Applications
Safety
Reagents & Buffers
Ammonium
Beer's Law
Biological membranes
Chlorine (Free)
Chlorine (Total)
Crystal Violet
Enzymes
Ethanol
Fats/Oils
Glucose
Halogenoalkanes
Hill Reaction
Iodination of propanone
Iodine Clock Reaction
Iron
Manganese in Steel
Nitrate
Nitrite
Phosphate
Population growth
Protein
Thiosulphate/HCl
Vitamin C
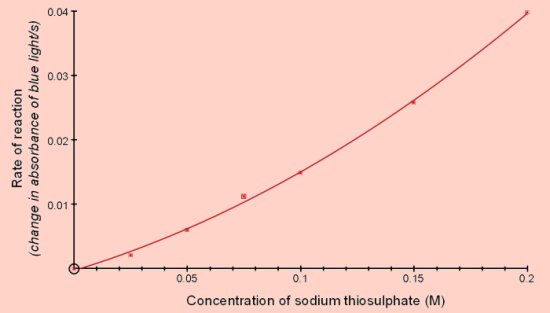
Reaction of sodium thiosulphate with acidThis is a well-known reaction found in most chemistry books and often carried out at GCSE level. Sodium thiosulphate reacts with acid to produce sulphur which is deposited in the solution causing it to become opaque.
The colorimeter can be used to measure the rate of this reaction since the increasing opacity scatters the light passing through.
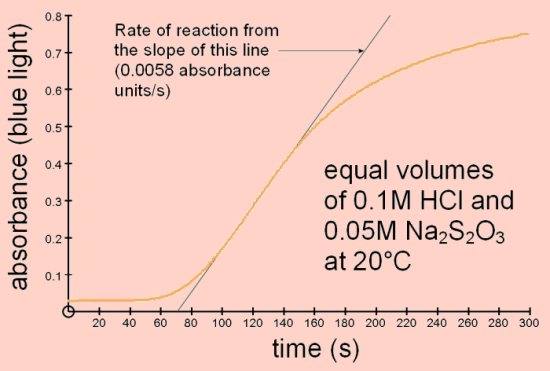
This investigation can be carried out with small volumes and low concentrations of the reactants.
|