Reactivity of Halogenoalkanes
In this experiment different halogenoalkanes
(haloalkanes, alkyl halides) are treated with silver nitrate in a mixture of
ethanol and water. The bond attaching the halide to the alkane is broken in a
reaction with water, releasing the halide which reacts with silver nitrate
forming a precipitate of silver halide. The rate of appearance of the precipitate
can be followed using the colorimeter.
R-Hal + OH- → R-OH + Hal-
Ag+ NO3- + Hal- → Ag+
Hal-
The rate of the reaction depends on the strength
of the carbon-halogen bond in the halogenoalkane, the stronger this is the longer
it takes for the precipitate to form.
|
There should be no need to use concentrations of any reagents that pose a
safety risk or require warning labels.
Halogenoalkanes should be dispensed in a
fume cupboard taking appropriate precautions but the working solutions contain
only low concentrations in ethanol.
|
|
Silver nitrate solutions equal to or stronger than 0.2 mol
dm-3 but weaker than 0.5 mol dm-3 should be labelled
"irritant".
|
 |
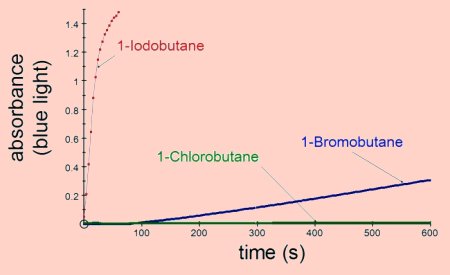
The most reactive halogenoalkanes are those forming the weakest bonds with
carbon, e.g. iodobutane is more reactive than bromobutane which is more reactive
than chlorobutane.
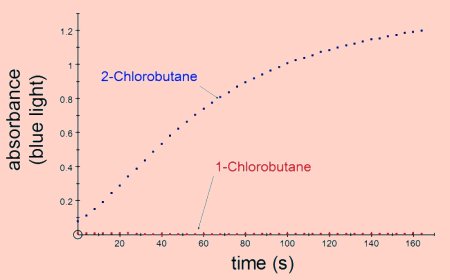
Primary halogenoalkanes are less reactive than secondary which are less reactive than
tertiary e.g. 1-chlorobutane is less reactive than 2-chlorobutane.
Using the colorimeter allows reaction rates to be measured so the effects of
concentrations, temperature, etc can be investigated.
|
The results shown above were obtained by adding 1cm3 of 0.1 mol dm-3 silver nitrate to 2cm3 of ethanol containing 0.1cm3 of the halogenoalkane. All the results were obtained at room temperature.
|
 |
Most disposable plastic cuvettes will become brittle and shatter if filled with ethanol. Use glass cuvettes or test-tubes
|
 |
|