Measurement of free chlorineThe method described here can be used to determine the free chlorine content of unknown solutions such as swimming pool water and is sensitive enough to measure the chlorine content of most domestic tap water When water is treated by chlorination, either in swimming pools or in the treatment of drinking water, it is present mainly as hypochlorous acid (HOCl). Depending on the pH there are also varying amounts of chlorine (Cl2) and hypochlorite ions (ClO-). These are the free active chlorine compounds in the water as opposed to the bound chlorine that has reacted with other compounds such as ammonia. The free chlorine compounds react with potassium iodide to release iodine.
The intensity of the colour produced can be measured using the colorimeter (blue light) and compared with standards of known concentration to measure free chlorine. The sensitivity of this method is increased by adding soluble starch solution which reacts with the iodine released giving an intense blue colour that can be measured using the red or green light on the colorimeter.
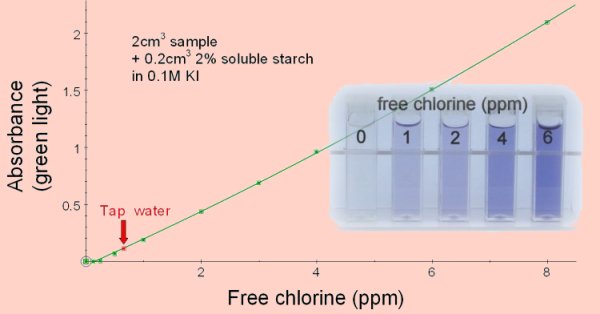
Add 2cm3 of the sample to be tested to 0.2cm3 of a 2% solution of soluble starch in 0.1M potassium iodide solution. Read the absorbance with green light. Standard solutions can be prepared from household chlorine bleaches which typically contain 3-6% sodium hypochlorite (NaClO). We used ‘Milton’ sterilising fluid which is 2% sodium hypochlorite. To prepare a solution containing 8ppm (8mg/l) chlorine the Milton was diluted 1/100 then 4.2cm3 of this was added to 80 cm3 distilled water. This was acidified by the addition of 0.5cm3 of ethanoic acid and made up to a final volume of 100cm3. For other sources of chlorine divide 0.084 by the percentage concentration of sodium hypochlorite. This volume (cm3) in 100cm3 of distilled water acidified with 0.5cm3 ethanoic acid will give a chlorine concentration of 8ppm (8mg/l). (This is best done in two steps, initially a 1% dilution so that a reasonable volume can be measured in preparing the final dilution.)
|
