|
In acid solution iodine reacts with propanone according to the reaction below.
|
CH3COCH3(aq)
|
+ |
I2 (aq)
|
→ |
CH3COCH2I(aq)
|
+ |
H+(aq)
|
+ |
I-(aq)
|
| colourless |
|
yellow/brown
|
|
colourless |
|
|
colourless |
The reaction can be followed using a colorimeter to measure the disappearance of the yellow/brown iodine colour.
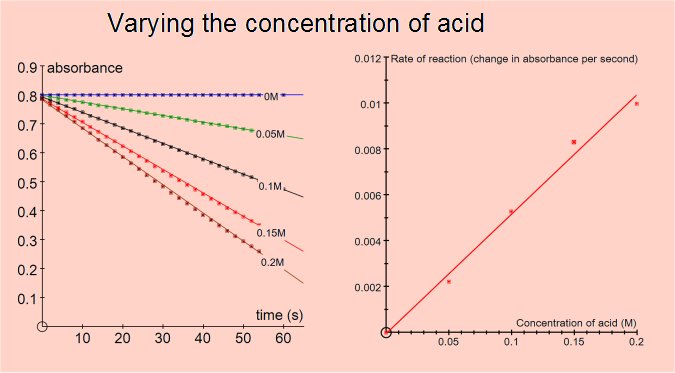
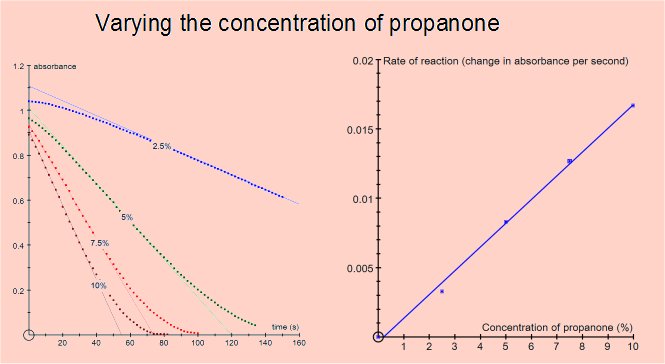
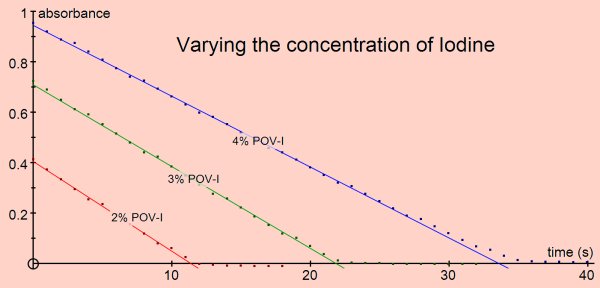
The rate of the reaction can be shown to be independent of the concentration of iodine (i.e. zero order for iodine) and directly proportional to the concentrations of propanone and acid (i.e first order).
The rate law for the reaction can be expressed as rate = k[CH3COCH3][H+]
|
The reagent concentrations used in the
examples illustrated here are relatively safe. Good laboratory practice should be
followed at all times. Students and teachers should complete risk assessments.
Solid iodine is corrosive and stock
solutions need to be prepared with care. Solutions greater than 1M should be
labelled ‘Irritant’ but the maximum concentration used here is less than 0.1M. We
recommend using commercial Povidone-Iodine instead of iodine in potassium iodide.
This gives good results at much lower concentrations of acid and propanone.
|
|
Sulphuric acid between 0.5 and 1.5M should be
labelled ‘Irritant’
|
 |
|
Propanone is highly flammable and MUST NOT be used near a
naked flame, is irritating to the eyes, degreases the skin and the vapour
can cause drowsiness or dizziness.
|

|
At room temperature (approx 20°C) the following mixture in a standard 4cm3 cuvette gave a linear drop in absorbance for 1 to 0A in about 1 minute.
-
2.5 cm3 10% propanone in 0.2M H2SO4
-
0.5 cm3 5% solution of povidone-iodine
Varying the concentration of acid or propanone gave reaction rates consistent with the reaction being first order for either reactant. Varying the iodine concentration did not affect the rate, the reaction is zero order for iodine.
 Click to download a pdf file about this investigation.
Click to download a pdf file about this investigation.
|





