EthanolUnder acidic conditions ethanol is oxidised to acetaldehyde by potassium dichromate.
The acetaldehyde is itself oxidised to acetic acid.
As the dichromate oxidises the reactants it is reduced to trivalent chromium Cr3+ which is green. The intensity of the green colour can be measured with the colorimeter and compared to known concentrations to measure the concentration of ethanol in the original sample. It should be noted that other organic molecules can also be oxidised by dichromate with similar results, though the only example we have found where this is significant is the oxidation of glucose. The amount of ethanol in a growing culture of yeast can be measured but a small proportion of the green colour obtained will come from the presence of glucose. You can compensate for this by measuring the amount of glucose using DNSA and adjusting accordingly.
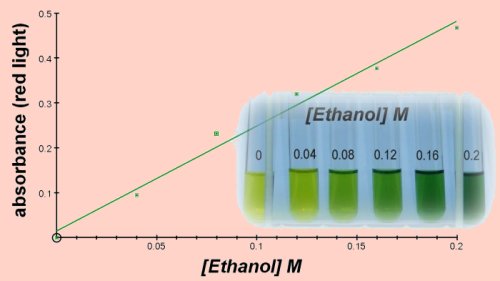
The method employed here used 0.5cm3 of sample to which was added 1cm3 of 1M H2SO4 containing 2% potassium dichromate. Incubation was at 50°C for 30 minutes. The colour change can be measured at this point or after the addition of 2cm3 of 2M NaOH (corrosive) to remove the remaining dichromate and reveal the green chromium Cr3+.
|



