Measurement of iron (Fe3+)
Iron(III) ions react with thiocyanate ions, SCN- giving an intense blood red solution containing the ion
[Fe(SCN)(H2O)5]2+.
The colour is easily detected and measured with the colorimeter and is an extremely sensitive test for the presence of small amounts of iron. A good, linear standard curve is obtained in the range 0-0.25M in a weakly acid solution.
The stoichiometry of the reaction can be investigated using Job’s method of continuous variation.
One application of this method could be to measure the amount of iron in a breakfast cereal since many of these are fortified with added iron.
 |
Potassium thiocyanate is harmful and particular care must be taken
not to add the solid to acid as it will release cyanide gas.
For the experiments suggested here the volumes used are relatively small and no
more than a few cm3 of 0.5M solution need be prepared.
|
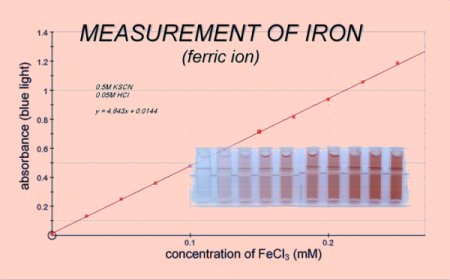 |
The final concentrations in the
cuvette
were- 0.5M potassium thiocyanate (KSCN)
- 0.05M hydrochloric acid (HCl)
-
Iron(III) chloride (Ferric chloride FeCl3) as shown on the x-axis.
|
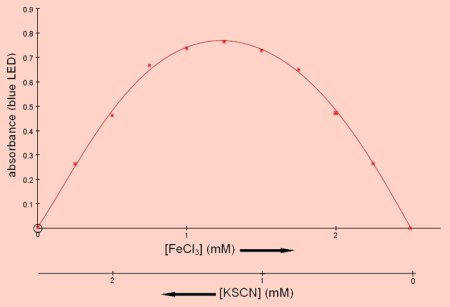
Job's method for determining reaction stoichiometry
Job’s method of continuous variation can be used to determine the stoichiometry of
the reaction between potassium thiocyanate and ferric chloride. The total number
of moles of reactants remains constant throughout a series of mixtures but the mole
fraction of each reactant varies. The symmetrical curve obtained shows that the red
complex contains an equal number of ferric and thiocyanate ions.
In addition to
the reactants indicated on the x-axis all reaction mixtures contained 0.5M HCl.
|