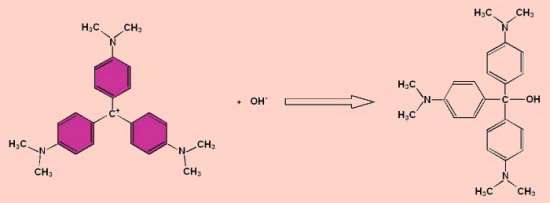
Decomposition of Crystal Violet
Crystal violet (aka gentian violet and numerous alternative trade names) reacts with alkali to form a colourless compound as shown below.
The reaction can be followed using a colorimeter to measure the disappearance of the violet colour.
 |
Solutions of crystal violet must be prepared with care since the material is harmful if swallowed, a severe eye irritant and there is limited evidence that it may cause cancer.
The dilute solutions of crystal violet used for the investigations here do not constitute a hazard (final concentration in the cuvette typically 4μg per cm3).
Solutions of sodium hydroxide between 0.05M and 0.5M should be labelled ‘Irritant’. Concentration greater than 0.5M should not be needed and it is not difficult to achieve good results with concentrations lower than 0.05M.
|
 |
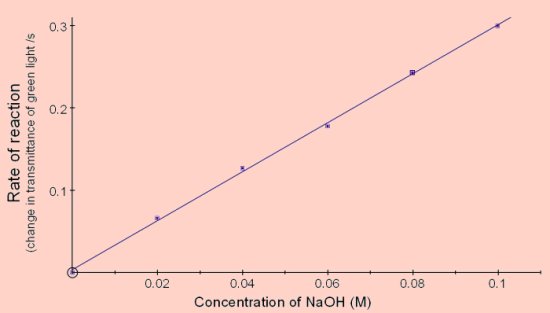
Transmittance vs Time
The results shown in below were obtained using cuvettes containing 4cm3
2cm3 of NaOH at twice the final concentration shown was placed in the cuvette and the reaction started by adding 2cm3 of crystal violet solution containing 16μg of crystal violet (8mg per litre prepared by dilution).
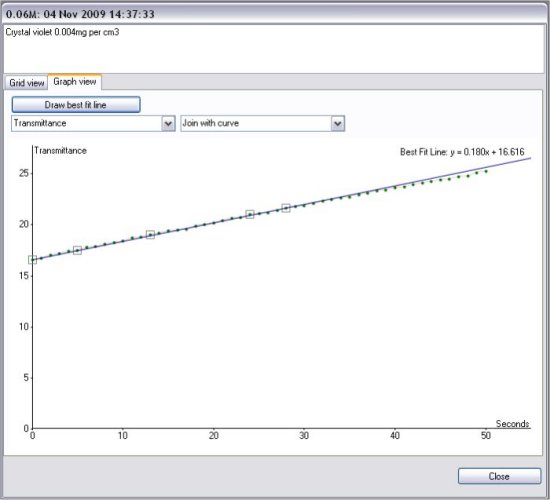
Effect of NaOH concentration
|