Saturation of Fats and OilsThe degree of saturation of fats or oils is usually determined by the iodine number, the number of grams of iodine that will react with 100g of the fat. The double bonds in unsaturated fats will react with iodine so the lower the saturation the higher the iodine number. Iodine number can be determined accurately by treating the fat with iodobromine, converting unreacted iodobromine to iodine using potassium iodide, then titrating the iodine with sodium thiosulphate. A simpler method of getting a rough estimate of the relative saturation of different fats and oils is to mix the fat with iodine solution. Some of the iodine will be removed by reaction with double bonds and the remaining iodine can be estimated using the colorimeter after reacting it with starch solution. We have used this method to compare coconut butter, (highly saturated, iodine number 8-10), olive oil, (moderately saturated, iodine number 75-95), and sunflower oil, (highly unsaturated, iodine number 130-145). Using these three as standards other fats and oils could be compared and their iodine numbers estimated.
Comparison of three oils
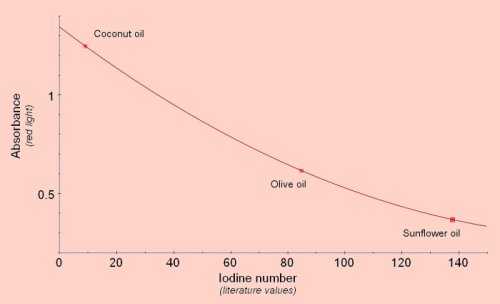
Add 1cm3 of the fat or oil to 5cm3 of iodine solution (stock solution diluted 1+4 with water). Shake vigorously for about 1 minute, (make sure each sample is given the same amount of shaking). Let the contents of the tube stand so that the oil layer will separate and float on the water layer, then remove 0.2cm3 from the lower, water layer and add it to 3cm3 of a 1% soluble starch solution. The remaining iodine will react giving a blue/black colour. Read absorbance using red light.
|