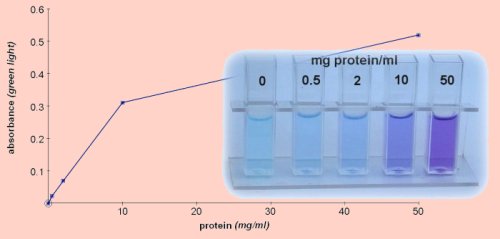
Biuret test for protein
Biuret reagent reacts with peptide bonds, turning from blue to purple. Unlike the Bradford test it will give equally good results with any protein, but it is unable to detect the low concentrations
that can be achieved with the Bradford test.
A qualitative test can be performed simply by adding equal quantities of 1% sodium (or potassium) hydroxide and a 1% solution of copper
sulphate to the sample. If the solution turns purple it means that protein is present.
The results described here use quantitative Biuret solution which will give a good estimate of protein concentrations in the range 0.1-10 mg/cm3.


|
Biuret reagent contains 0.75M sodium hydroxide.
THE USE OF EYE PROTECTION MUST BE STRICTLY
ENFORCED
In the event of eye contact flood the eye gently with running water for ten minutes and seek medical attention.
|
 |
Biuret reagent
|
Solution 1
|
Copper sulphate.5H2O
Sodium potassium tartrate
|
0.75g
0.3g
|
|
Dissolve in 250cm3 H2O
|
Solution 2
|
| Sodium hydroxide |
15g |
|
Dissolve in 150cm3 H2O
Add 2 to 1 mixing thoroughly and make up to 500cm3.
If a precipitate forms 0.5g potassium iodide can be added
|
| Label the stock solution 'CORROSIVE' |

|
-
To perform the quantitative test add 2 cm3 of Biuret reagent to 0.5 cm3 of sample.
- Allow the mixture to stand for 10 minutes then read absorbance in green light, (~565nm).
Similar results were obtained using either powdered egg white or gelatine as the protein standard
 Click to download a pdf file containing this information.
Click to download a pdf file containing this information.
|