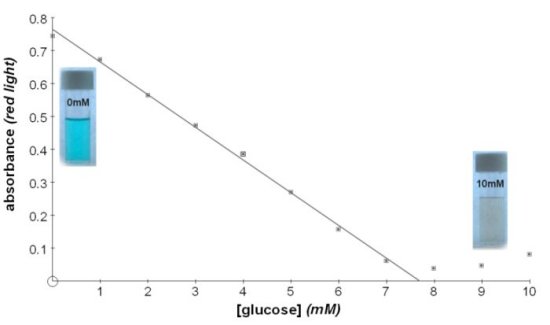
Benedict's TestQualitative or quantitative test for reducing sugarsBenedict’s solution reacts with reducing sugars on heating and reduces the Cu(II) ion to Cu(I) producing a precipitate of red copper oxide. The resulting colour change depends on the type and concentration of sugar, so this test has been used semi-quantitatively to indicate approximate concentrations. An alternative version of Benedict's reagent for quantitative testing (QBS) contains potassium thyocyanate and does not form red copper oxide. Instead the presence of reducing sugar is measured by the loss of the blue colour of copper sulphate and a white precipitate is formed which will settle out or can be removed by filtration before colorimetric determination of the filrate. Using a colorimeter and either version of Benedict's reagent you can obtain accurate, fully quantitative determinations of concentration down to 0.001M, (180µg of glucose/cm3). This is about 5 times lower than the concentrations detectable with test strips. Lower concentrations can be detected rather more easily and in smaller volumes using DNSA
Benedict's reagent
You can use Benedict's reagent in a quantitative test....
At low concentrations of reducing sugar there will be unreacted copper sulphate left. Read absorbance using red light. At higher concentrations there will be increasing amounts of red copper oxide in the filtrate. Read absorbance using blue light Quantitative Benedict's reagent This solution contains potassium thiocyanate and does not give a red precipitate on boiling. The amount of reducing sugar present is measured by the disappearance of the blue colour of copper sulphate.
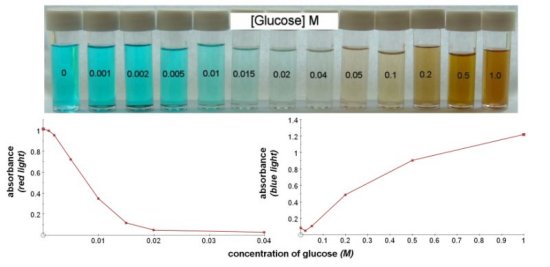
Quantitative Benedict's solution
|
||||||||||||||||||||||||||||||