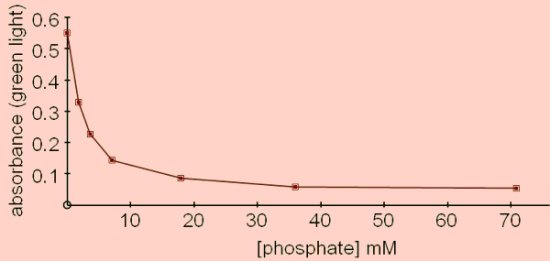
PhosphataseA phosphatase is an enzyme that releases a phosphate group from its substrate by hydrolysis. The assay method described here is for an acid phosphatase, (i.e. a phosphatase with an optimum pH below 7 - EC 3.1.3.2), that can readily be obtained from germinated mung beans, (beansprouts), though other sources could be investigated. To measure the activity of the enzyme a good substrate is phenolphthalein phosphate which is colourless until it is hydrolysed to phenolphthalein. Phenolphthalein is pink in alkaline solution and the absorbance can be measured using the colorimeter and green light. Adding the reaction mixture to an alkali also serves to stop the reaction. 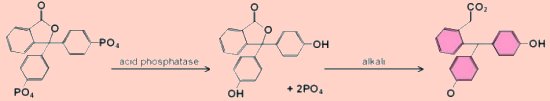 This reaction is particularly suitable for studying the effects of end product inhibition since the addition of small amounts of phosphate has a marked effect on the rate of the reaction.
End product inhibition
The buffer was citric acid-sodium citrate pH6 supplemented with phosphate buffer pH6 to give a range of phosphate concentrations between 0 and 80mM. The reaction mix was incubated for 10 minutes at 25°C then stopped by adding 0.5cm3 of the reaction mix to 2.5cm3 of 10% sodium carbonate.
Enzyme extraction from beansprouts
(Alternatively add 10cm3 of citric acid-sodium citrate buffer pH6. Do not use a buffer containing phosphate. Filter the mixture through several layers of muslin and store refrigerated. The extract can be centrifuged to remove debris and obtain a clear extract but this is optional. Reaction mixture
The end point should turn pink on addition to sodium carbonate. Incubate for 10 minutes at 25°C then add 0.5cm3 of the reaction mixture to 2.5cm3 10% sodium carbonate solution. Read absorbance using green light. End product inhibition
For example:
|