NeutraseNeutrase is the registered trade name of an endoprotease from Bacillus amyloiquefaciens manufactured by Novozymes and available from NCBE. . At neutral pH it hydrolyses internal peptide bonds. The activity of neutrase can be assayed using dried skimmed milk powder such as Marvel or any supermarket brand low-fat milk powder. As the reaction proceeds the milk proteins are broken down and rendered soluble, so the cloudy suspension of insoluble proteins gradually becomes clear.
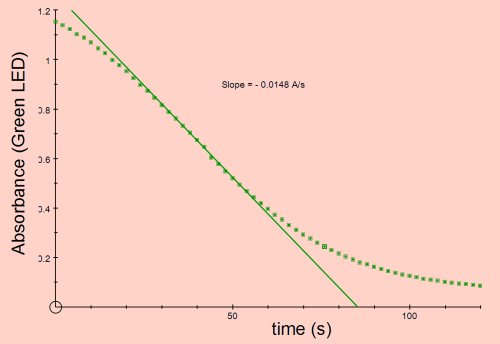
Absorbance vs Time
The rate of the reaction shown below is the slope of the line in the region between absorbance 0.8 and 0.5, given as
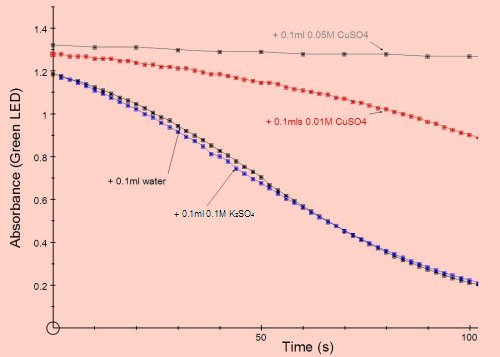
Inhibition by metal ions. Many heavy metal ions are non-competitive enzyme inhibitors. The graphs below show typical sets of results obtained with salts of copper and lead.
The inhibitor was added in 0.1cm3 to a 3cm3 total reaction mix.
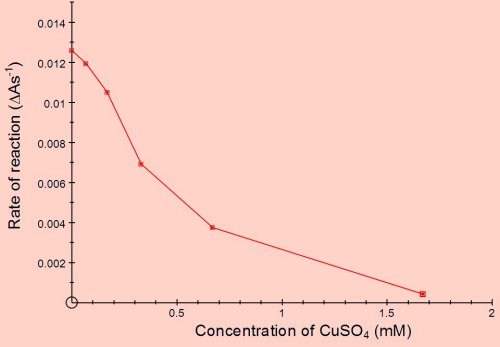
The graph below shows the effect of concentration of copper sulphate on the rate of reaction. The copper sulphate was added in 0.1cm3 of water and the concentrations are the final concentrations in the 3cm3 cuvette.
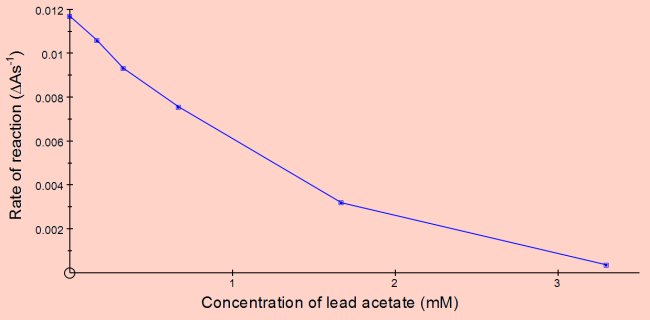
Inhibition of neutrase by lead acetate. The graph below shows a set of results with lead acetate obtained using the same method as in the previous example.
The experiments shown above were all carried out at room temperature using a reaction mixture containing:
|