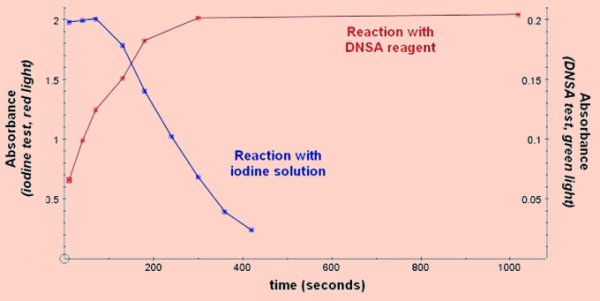
β-amylaseβ-amylase breaks every second bond in starch producing maltose, but cannot pass the branches (1,6 linkages) in amylopectin so the blue/black iodine complex with soluble starch is only partially reduced. The appearance of maltose is usually used to measure the rate of reaction, but the disappearance of the blue iodine complex can be used if amylose is the substrate. Sweet potato is an excellent source of β-amylase, (EC 3.2.1.2). Enzyme activity can be shown using soluble starch as the substrate and DNSA or Benedicts reagent to measure the maltose produced. There is some reduction in the colour with iodine solution during the reaction which can be measured with the colorimeter. An alternative method is to use amylose prepared from potato starch as the substrate as this is completely broken down to maltose, (since there are no branches in the chains to stop the progress of the enzyme), and the blue colour obtained with iodine solution will disappear completely. If you have a centrifuge the β-amylase from sweet potato can easily be partially isolated by precipitation with ammonium sulphate, (or completely isolated and crystallised if you are prepared to go through a rather lengthy series of precipitation and resuspension stages). Sweet potato β-amylase is a stable enzyme and will keep well in a refrigerator.
Enzyme extraction
The extract is filtered through several layers of butter muslin. If a centrifuge is available it can be used to remove cell debris but the extract will give good results without this stage. The extract will keep for several weeks refrigerated without much loss of activity. Reaction mixture
The buffer/substrate mixture should be allowed to equilibrate to the reaction temperature before being added to the enzyme. Using soluble starch or amylose as the substrate and measuring the production of maltose. Using amylose as the substrate and measuring the colour of the blue complex formed between amylose and iodine. Preparation of amylose from potato starch
Katakuriko flour used in Japanese cooking is potato starch and works well with this method. Amylose gives a bright blue colour with iodine solution. Purification of β-amylase by precipitation with ammonium sulphate
The method described below will concentrate and partially purify β-amylase from sweet potato, but much greater degrees of purification are possible using narrower ranges of ammonium sulphate concentration, (and precipitation with propanone).
Powdered ammonium sulphate should be used.
|


