α-amylaseα-amylase randomly breaks the bonds between glucose residues in starch. The product is a mixture of small saccharides including maltose. The appearance of reducing sugars can be measured, but the easiest method of following the reaction is to measure the disappearance of the blue/black starch-iodine complex. α-amylase (EC 3.2.1.1) is present in saliva. Salivary amylase, (ptyalin), is readily available and is considered safe to use when simple precautions are followed. There are a number of different bacterial and fungal α-amylases available in liquid or powdered form. The reaction between soluble starch and α-amylase can be followed by monitoring the disappearance of the blue/black starch-iodine complex or the appearance of reducing sugars using DNSA reagent or Benedict’s reagent . Salivary amylase is good for showing the effects of temperature and pH on enzyme activity as it is relatively sensitive to both conditions, though the optimum temperature is considerably higher than is usually given in school textbooks.
Absorbance vs Time - 30°C, pH7
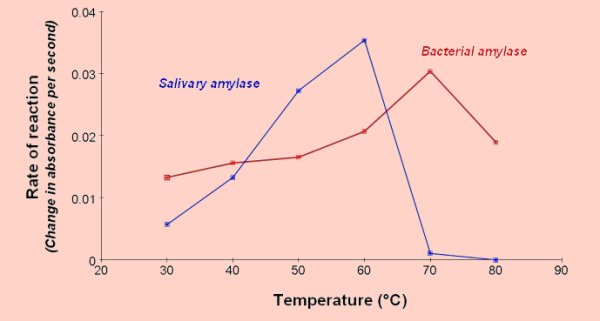
Effect of temperature
Effect of pH
To collect salivary amylase
Reaction mixture
A suitable reaction protocol for α-amylase is as follows;
Before adding it to the enzyme the substrate/buffer mixture should be equilibrated to the reaction temperature; At 30 second intervals remove 0.1cm3 of the reaction mixture and add it to 3cm3 of iodine solution, (2% iodine stock solution in 0.1M HCl) Read absorbance using red light (635nm).
|
||||||||||||